Question Text
Unit No. 5 CHEMICAL REACTIONS
- What is meant by physical change?
Ans: In a physical change, only the shape, size, or physical state of material changes.
Examples:
The change of water into ice, water, and water vapours are examples of physical changes.
2. What is a chemical change? Ans: In a chemical change, a material changes into a new material with different chemical compositions and properties.
Examples:
The burning of wood and rusting of iron are chemical changes.
3. Give some examples of chemical change.
Ans: Rusting of iron, burning of paper, cooking of food,
If we pass electricity through acidified water (H2O), it will turn into hydrogen (H) and oxygen (O). It is a chemical change.
4. What is a chemical reaction?
Ans: The process during which a substance changes into a new substance with different chemical compositions and properties is called a chemical reaction. Or
The process of chemical change is called a chemical reaction.
5. Give some examples of chemical reactions.
Ans: Respiration, photosynthesis, and burning are examples of chemical reactions.
6. What are the reactants?
Ans: Substances that take part in a chemical reaction are called reactants.
7. What are the products?
Ans: Substances formed due to the chemical reaction are called products.
8. What is meant by fermentation reaction?
Ans: The process of chemical change by microorganisms is called a fermentation reaction. The conversion of milk into yogurt is an example of fermentation
9. What is a chemical equation? Or Define chemical equations.
Ans: A chemical equation is the representation of a chemical reaction in terms of symbols, formulae, and signs. An arrow separates the reactants and products.
Example: CaCO3 + Heat => CaO + CO2
10. What is meant by a balanced chemical equation?
Ans: The chemical equation is balanced if the number of atoms of each element on both sides of the equation is equal.
Example: CaCO3 + Heat => CaO + CO2
11. What is meant by an unbalanced chemical equation?
Ans: The chemical equation is unbalanced if the number of atoms of each element on both sides of the equation is not equal.
Example : Mg + O2 => MgO
12. Define the law of conservation of mass.
Law of Conservation of Mass
During a chemical reaction, the products’ total mass equals the total mass of the reactants.
This law states that mass is neither created nor destroyed during a chemical reaction but changes from one form to another.
13. What are the addition reactions?
Ans: The chemical combination of two or more substances to form one compound is called an addition reaction.
14. Give two examples of addition reactions.
Ans:
1. Hydrogen and Oxygen form water.
2H2 + O2=> 2H2O
2. Sodium and Chlorine form sodium chloride (table salt).
2Na + Cl2 => 2NaCl
15. What is decomposition?
Decomposition
A compound splits into two or more simple substances is called decomposition. Usually, heat is required to bring about the decomposition of compounds.
ii. Sodium and Chlorine form sodium chloride (table salt).
2Na + Cl2 => 2NaCl
Ans:
i. When we give heat to potassium chlorate, it splits into potassium chloride and oxygen.
2KClO3 + Heat => 2KCl + 3O2
ii. When we give heat to calcium carbonate, it changes into calcium oxide and carbon dioxide gas.
CaCO3 + Heat => CaO + CO2
17. How many types of change are on the basis of the change in energy?
Ans: On the basis of the change in energy, chemical reactions can be classified into two
types.
- Exothermic Reactions
- Endothermic Reactions.
Ans: Exo means ‘outside’ and therm means ‘heat’. The chemical reactions during which heat is given out (evolved) are called exothermic reactions.
Ans:
- Burning of natural gas
- The chemical reaction of calcium oxide and water
Ans: Endo means inside. During endothermic reactions, heat is absorbed.
CaCO3 + heat => CaO + CO2
Example 2. The formation of nitric oxide from nitrogen and oxygen is an endothermic reaction.
N2 + 2O2 + Heat => 2NO2
22. What is the importance of exothermic reactions in daily life? Or Write uses of exothermic reactions.
Exothermic reactions have great importance in our daily life.
- It is used to cook food in our homes.
- It is used to move the piston in engines and the force of the piston moves the vehicle.
- The heat produced during the digestion of food in our bodies keeps us warm and alive.
Addition reaction:
The chemical combination of two or more substances to form one compound is called an addition reaction.
Hydrogen and Oxygen form water.
2H2 + O2 => 2H2O
Decompositions:
A compound splits into two or more simple substances is called decomposition. Usually, heat is required to bring about the decomposition of compounds.
When we give heat to potassium chlorate, it splits into potassium chloride and oxygen.
2KClO3 + Heat => KCl + 3O2
Balanced Chemical Equation:
The chemical equation is balanced if the number of atoms of each element on both sides of the equation is equal.
Unbalanced Chemical Equation:
The chemical equation is unbalanced if the number of atoms of each element on both sides of the equation is not equal.
Exothermic Reactions:
Exo means outside’ and therm means ‘heat’. The chemical reactions during which heat is given out (evolved) are called exothermic reactions.
Endothermic Reactions:
Endo means inside. During endothermic reactions, heat is absorbed.
Ans: 1. Photosynthesis is a chemical reaction by which food is produced in the leaves of plants. This food is used by plants and animals.
2. Respiration is a chemical reaction that provides energy to our bodies.
The working rules for balancing a chemical equation
- Write the unbalanced equation and count the number of atoms of each element on both sides of the arrow.
- Work with one element at a time.
- Multiply the symbol or formula with suitable integers on that side of the equation where the number of atoms of a particular element is less.
- Repeat this process for all elements one by one.
- Balance diatomic molecules like H2, N2, etc. at the end.
28. Describe the applications of chemical reactions.
Burning, respiration, and photosynthesis are examples of chemical reactions.
- We burn methane (natural gas) gas to cook food in homes.
- Burning of petrol is used to move vehicles.
- The burning of fuel is used in industries to produce steam.
- During respiration, energy is produced. This energy is used to perform all body functions.
- The conversion of milk into yogurt is also a chemical reaction.
- Photosynthesis is a chemical reaction. During photosynthesis plants make food.
29. When coal burns, it leaves ash behind. Ash so produced is lighter than the coal which has burnt. Justify the decrease in mass in light of the law of conservation of mass.
Ans: According to the law of conservation of mass coal reacts with oxygen and changes into ash, carbon dioxide, and water vapors. Carbon dioxide and water vapors are released into the atmosphere and only ash has remained on the ground. Thus, the total mass of products remains equal to the total mass of reactants.
- Iron + Hydrochloric acid => Iron chloride + Hydrogen
- Calcium oxide + Carbon dioxide => Calcium Carbonate
- Carbon monoxide + Oxygen => Carbon dioxide
- Carbon dioxide + water → Glucose + Oxygen
- 2Mg + O2 => 2MgO
- CH4 + 2O2 => CO2 + 2H2O
- Fe + S => FeS
- N2 + 3H2 => 2NH3
- 2Na + Cl2 => 2NaCl
- Ca(HCO3)2 + 2HCl => CaCl2 + 2CO2 + 2H2O
- 2NaBr + Cl2 => 2NaCl + Br2
- 4Fe + 3O2 => 2Fe2O3
- 2NH4OH + H2SO4 => (NH4)2SO4 + 2H2O
- Zn + 2HCl => ZnCl2 + H2
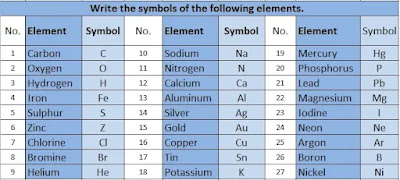
33. Write the symbols of the following elements.
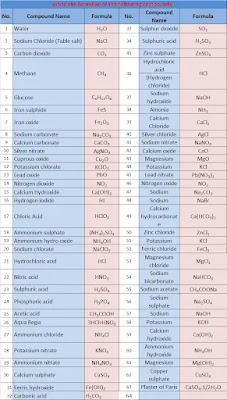
34. Write the formulae of the following compounds.
| Compound Name | Formula | No. | Compound Name | Formula | ||
| 1 | Water | H2O | 21 | Sulfur dioxide | SO2 | |
| 2 | Sodium Chloride (Table salt) | NaCl | 22 | Sulphuric acid | H2SO4 | |
| 3 | Carbon dioxide | CO2 | 23 | Zinc sulphate | ZnSO4 | |
| 4 | Methane | CH4 | 24 | Hydrochloric acid (Hydrogen chloride) | HCl | |
| 5 | Glucose | C6H12O6 | 25 | Sodium hydroxide | NaOH | |
| 6 | Iron sulphide | FeS | 26 | Amonia | NH3 | |
| 7 | Iron oxide | Fe2O3 | 27 | Calcium Chloride | CaCl2 | |
| 8 | Sodium carbonate | Na2CO3 | 28 | Silver chloride | AgCl | |
| 9 | Calcium carbonate | CaCO3 | 29 | Sodium nitrate | NaNO3 | |
| 10 | Silver nitrate | AgNO3 | 30 | Calcium oxide | CaO | |
| 11 | Cuprous oxide | Cu2O | 31 | Magnesium oxide | MgO | |
| 12 | Potassium chlorate | KClO3 | 32 | Potassium chloride | KCl | |
| 13 | Lead oxide | PbO | 33 | Lead nitrate | Pb(NO3)2 | |
| 14 | Nitrogen dioxide | NO2 | 34 | Nitrogen oxide | NO2 | |
| 15 | Calcium Hydroxide | Ca(OH)2 | 35 | Sodium carbonate | Na2CO3 | |
| 16 | Hydrogen iodide | HI | 36 | Sodium bromide | NaBr | |
| 17 | Chloric Acid | HClO3 | 37 | Calcium hydrocarbonate | Ca(HCO3)2 | |
| 18 | Ammonium sulphate | (NH4)2SO4 | 38 | Zinc chloride | ZnCl2 | |
| 19 | Ammonium hydro oxide | NH4OH | 39 | Potassium chloride | KCl | |
| 20 | Sodium chlorate | NaClO3 | 40 | Ferric chloride | FeCl3 | |
| 41 | Hydrochloric acid | HCl | 52 | Magnesium chloride | MgCl2 | |
| 42 | Nitric acid | HNO3 | 53 | Sodium bicarbonate | NaHCO3 | |
| 43 | Sulphuric acid | H2SO4 | 54 | Sodium acetate | CH3COONa | |
| 44 | Phosphoric acid | H3PO4 | 55 | Sodium sulphate | Na2SO4 | |
| 45 | Acetic acid | CH3COOH | 56 | Sodium hydroxide | NaOH | |
| 46 | Aqua Regia | 3HCl+HNO3 | 57 | Potassium hydroxide | KOH | |
| 47 | Ammonium chloride | NH4Cl | 58 | Calcium hydroxide | Ca(OH)2 | |
| 48 | Potassium nitrate | KNO3 | 59 | Ammonium hydroxide | NH4OH | |
| 49 | Ammonium nitrate | NH4NO3 | 60 | Magnesium hydroxide | Mg(OH)2 | |
| 50 | Calcium sulphate | CaSO4 | 61 | Copper sulphate | CuSO4 | |
| 51 | Ferric hydroxide | Fe(OH)3 | 62 | Plaster of Paris | CaSO4.H2O | |
| 63 | Carbonic acid | H2CO3 |